Article Text
Abstract
We have evaluated dietary recommendations for people diagnosed with familial hypercholesterolaemia (FH), a genetic condition in which increased low-density lipoprotein cholesterol (LDL-C) is associated with an increased risk for coronary heart disease (CHD). Recommendations for FH individuals have emphasised a low saturated fat, low cholesterol diet to reduce their LDL-C levels. The basis of this recommendation is the ‘diet-heart hypothesis’, which postulates that consumption of food rich in saturated fat increases serum cholesterol levels, which increases risk of CHD. We have challenged the rationale for FH dietary recommendations based on the absence of support for the diet-heart hypothesis, and the lack of evidence that a low saturated fat, low cholesterol diet reduces coronary events in FH individuals. As an alternative approach, we have summarised research which has shown that the subset of FH individuals that develop CHD exhibit risk factors associated with an insulin-resistant phenotype (elevated triglycerides, blood glucose, haemoglobin A1c (HbA1c), obesity, hyperinsulinaemia, high‐sensitivity C reactive protein, hypertension) or increased susceptibility to develop coagulopathy. The insulin-resistant phenotype, also referred to as the metabolic syndrome, manifests as carbohydrate intolerance, which is most effectively managed by a low carbohydrate diet (LCD). Therefore, we propose that FH individuals with signs of insulin resistance should be made aware of the benefits of an LCD. Our assessment of the literature provides the rationale for clinical trials to be conducted to determine if an LCD would prove to be effective in reducing the incidence of coronary events in FH individuals which exhibit an insulin-resistant phenotype or hypercoagulation risk.
- nutritional sciences
- cardiovascular diseases
- disease management
- evidence-based practice
- integrative medicine
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- nutritional sciences
- cardiovascular diseases
- disease management
- evidence-based practice
- integrative medicine
Introduction
In a compelling editorial, Steven Nissen, Chair of the Department of Cardiovascular Medicine at the Cleveland Clinic,1 stated ‘current and past US dietary guidelines represent a nearly evidence-free zone’ and that as a consequence of the promotion of ‘low-fat, low cholesterol diets … Type 2 diabetes grew into an epidemic’. He asserted that dietary recommendations should be ‘based on the same quality of evidence that we demand in other fields of medicine’. We have applied his call for evidence-based dietary guidelines to the routine dietary recommendations that are given to individuals diagnosed with familial hypercholesterolaemia (FH), a genetic condition characterised by an elevated level of low-density lipoprotein cholesterol (LDL-C), which is associated with an increased incidence of coronary heart disease (CHD).2 The issue we have addressed is whether dietary recommendations for FH, which promote the cardiovascular benefits of a low cholesterol, low saturated fat diet, are based on strong empirical support or exist, in the context of Nissen’s editorial, in an ‘evidence-free zone’.
Historical perspective on dietary recommendations for FH
In 1939, Muller3 provided the first documentation of premature heart disease in people diagnosed with FH, in conjunction with dietary guidance. He recommended FH patients consume a diet ‘poor in cholesterol’ without any ‘yolk of egg, butter, cream, fat milk or any fat of animal origin’. However, in a note of caution, he stated there was no empirical basis ‘to formulate any opinion in regard to the effects (of the diet)’. Fifty years later, Connor and Connor4 praised Muller for recommending ‘dietary cholesterol and animal fat as necessary restrictions in patients with FH’. Their praise of Muller’s dietary recommendations for FH, however, did not address his concerns that the presumed benefits of the diet had not been confirmed in a randomised controlled trial (RCT). Indeed, when the Connors praised Muller’s guidelines for FH there had not been a single RCT reporting coronary event benefits from the diet that had become the standard of care for FH individuals.
Dietary guidance for FH individuals has not changed in the three decades since the Connors praised the value of a low saturated fat, low cholesterol diet. Examples of contemporary recommendations for FH include DeBeasi,5 who urged FH individuals to consume ‘lean cuts of meat’ and to ‘remove skin from poultry, select reduced fat cheese and milk, and avoid coconut and palm oils, butter, sour cream, lard, and ghee’. Dietary recommendations in the 2018 AHA cholesterol guidelines stated that FH individuals should follow an LDL-lowering ‘heart healthy’ diet, which limits animal and vegetable sources of saturated fat, emphasising consumption of ‘low-fat dairy products and low-fat poultry (without the skin)’ … and non-tropical vegetable oils.’ 6
Despite the consensus that FH individuals should follow a diet low in saturated fat and cholesterol, there is a conspicuous absence of dietary RCTs with FH individuals as subjects with evidence of benefits toward cardiovascular endpoints. Indeed, a recent Cochrane Collaboration’s comprehensive review of 15 dietary RCTs stated ‘No conclusions can be made about the effectiveness of a cholesterol-lowering diet … for FH, for the primary outcomes’, which referred to the absence of findings on the effects of cholesterol lowering diets on the incidence of heart disease and mortality in FH. The authors suggested ‘There is a need for long-term trials with parallel group design to assess the potential benefits and harms of a cholesterol-lowering diet’.7
This historical perspective illustrates the absence of an evidence-basis for dietary recommendations for FH individuals. Even worse, the emphasis on a low-fat diet (LFD) may result in FH people consuming carbohydrate-dense food, which is potentially counterproductive, in that this diet may exacerbate an insulin-resistant phenotype.8–10 Given that a subset of FH individuals are at a greater risk for developing CHD than the general population, their dietary guidelines should be based on strong evidence.
Historical perspective on the diet-heart hypothesis
The basis of Muller’s recommendation that FH individuals restrict saturated fat intake came to be referred to as the diet-heart hypothesis, which postulated that consumption of saturated fat raises serum cholesterol levels, which increases one’s risk of developing CHD. From its inception, the utility of the diet-heart hypothesis has been repeatedly challenged. In one of the first of many critiques, Yudkin flatly dismissed it, stating ‘such a simple hypothesis cannot be sustained’.11 Yudkin displayed sophistication rarely seen in 1950s researchers by concluding the ‘evidence points to a multifactorial aetiology of cardiac infarction’, which he proposed included diet, mental stress, obesity, sedentary lifestyle and smoking. Contemporary researchers have expanded on Yudkin’s commentary to point out that the diet-heart hypothesis does not take into account the totality of dietary nutrients and lifestyle factors, such as smoking and sugar consumption, which contribute to the development of CHD.8 9 12–14
Despite numerous critiques of the diet-heart hypothesis,8 9 12–15 dietary guidance for FH remains entrenched in the view that a low saturated fat, low cholesterol diet will protect FH individuals from developing CHD. Contemporary FH diet recommendations are exemplified in a recent commentary by Gidding,16 who stated ‘A low saturated fat/low-cholesterol diet will lower cholesterol and improve outcomes in those with FH.’ However, Gidding conceded that his dietary recommendation for FH was largely speculative, with the caveat that ‘there is only a minimal literature on diet management in FH’.
Recently, DuBroff and de Lorgeril13 evaluated the diet-heart hypothesis in a comprehensive review of 28 RCTs that assessed the effects of dietary interventions on cardiovascular and/or mortality outcomes in non-FH individuals. Regarding the putative link of dietary cholesterol to coronary outcomes, they found only two trials that reported a mortality benefit of diet in the intervention group, and both of these trials showed no change in serum cholesterol levels with the diet intervention. DuBroff and de Lorgeril also reported there were eight RCTs that explicitly involved replacement of saturated fat with polyunsaturated fats, but none reported a mortality benefit and only two reported a reduction in cardiovascular events. Finally, they noted that two RCTs ‘reported increased mortality and/or cardiovascular events with cholesterol reduction’. In opposition to the core feature of the diet-heart hypothesis, DuBroff and de Lorgeril concluded ‘diets that replace saturated fat with polyunsaturated fat do not convincingly reduce cardiovascular events or mortality’.
In summary, current dietary recommendations for FH individuals are based on the unsupported diet-heart hypothesis. Therefore, routine dietary recommendations for FH individuals exist in an evidence-free zone.
Contemporary research on heart disease risk factors
Because LDL-C is elevated in FH and a subset of individuals with FH exhibit premature CHD, a diet-induced reduction of LDL-C has been assumed to serve as an effective surrogate marker for improved cardiovascular health. However, there is strong support for the view that LDL-C, in isolation, is a poor marker of risk for CHD in the general population, as well as in FH.17–20 Indeed, Bittencourt et al,21 recently commented on the finding of a substantial percentage of individuals with very high LDL-C (>190 mg/dL) who also had a zero Coronary Artery Calcium (CAC) score. Hence, despite their high LDL-C levels, these individuals with a zero CAC score had a very low risk for future coronary events. Moreover, a comprehensive review of studies on mortality rate in relation to LDL-C levels showed that people over 60 years of age with the highest LDL-C lived as long, or even longer, than those with low LDL-C.22 Therefore, it is of value to identify biomarkers other than LDL-C which are closely associated with CHD, and more importantly, are affected by dietary interventions which may be of benefit to FH individuals.
Atherogenic dyslipidaemia risk triad: triglycerides, high-density lipoprotein (HDL) and small, dense LDL
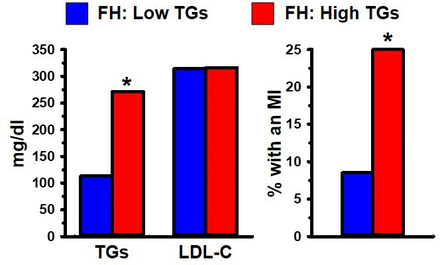
LDL-C is contained in heterogeneous particles which range in size and composition from a small, dense, triglyceride (TG) rich LDL (sdLDL) to a large, buoyant, cholesterol-enriched LDL (lbLDL). This distinction between LDL particle subclasses is important because sdLDL, unlike lbLDL, is a component of an atherogenic dyslipidaemia risk triad (ADRT), composed of elevated levels of TGs and sdLDL, in conjunction with low levels of HDL.8–10 23 Each of the three components of the ADRT, individually, has been associated with increased risk of CHD. For example, sdLDL, unlike lbLDL, is a unique marker of CHD risk, independent of LDL-C.24 Another study demonstrated that FH individuals, distinguished solely on the basis of having high TGs (>200 mg/dL), exhibited three times greater occurrence of a myocardial infarction (MI), compared with FH individuals with low TGs (<200 mg/dL).25 It is noteworthy that the association of high levels of TGs in FH with a high rate of MI occurrence was independent of their LDL-C levels (figure 1). Overall, the ADRT is a highly reliable measure of CHD risk in FH, as well as non-FH, individuals.
(Left) Heterozygous FH men with low (<200 mg/dL; blue) or high (>200 mg/dL; red) fasting plasma triglycerides (TGs). (Right) The group with high TGs had a significantly greater incidence of MI than the group with low TGs. Data from Moorjani et al 25. *Indicates p<0.05 compared with the relevant comparison group, based on statistical analyses in the original publications. FH, familial hypercholesterolaemia; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction.
Heterozygous FH individuals grouped according to whether they had symptomatic coronary heart disease (CHD+) or not (CHD−). The two groups did not differ significantly in their LDL-C levels but differed significantly in their Lp(a) levels. Data from Seed et al. 27 *Indicates p<0.05 compared with the relevant comparison group, based on statistical analyses in the original publication. FH, familial hypercholesterolaemia; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein a.
Lipoprotein a
Lipoprotein a (Lp(a)) is one of the most robust of all markers of CHD risk in FH and non-FH populations.26 Lp(a) contains a plasminogen-like glycoprotein, known as apolipoprotein (a), which is bound to the apolipoprotein B-100 of an LDL particle. Elevated levels of Lp(a) are more closely associated with CHD than is LDL-C. For example, Seed et al,27 showed that FH individuals with CHD had significantly greater levels of Lp(a) compared with FH without CHD; the association of Lp(a) with CHD in FH was independent of their LDL-C levels (figure 2).
Haemostatic balance between coagulation and fibrinolysis
A powerful influence on the development of CHD is the interplay between processes that promote clot formation (coagulation) and those that cause clots to lyse (fibrinolysis). There is extensive evidence, at cellular, metabolic and genetic levels of analysis, that the haemostatic balance in FH is shifted toward hypercoagulation. These findings were reviewed by Ravnskov et al,18 who found strong evidence of hypercoagulation, and not LDL-C, as a cause of CHD in FH. A subset of the literature is provided below.
Platelets from FH individuals are more sensitive than those of non-FH individuals to aggregate in response to epinephrine.28 29 This finding suggests that FH individuals would develop a greater thrombotic reaction to stress than non-FH individuals.
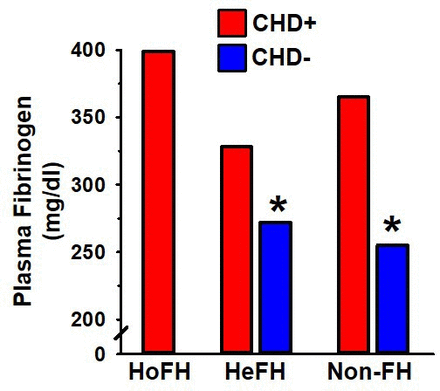
Extremely high levels of fibrinogen, a primary clotting factor and risk factor for CHD,30 are found in homozygous FH individuals, which have a high incidence of early CHD-related mortality.31 High levels of fibrinogen also distinguish the subset of heterozygous FH individuals (as well as non-FH) with CHD from those without CHD (figure 3).30 32
Genetic factors can influence haemostatic balance. For example, prothrombotic gene polymorphisms, such as prothrombin 20 210A, increase the risk of MI in the general population.33 FH individuals with the prothrombin 20 210A polymorphism exhibited more than twice the rate of coronary events as FH individuals without the polymorphism, an effect which was independent of their LDL levels.34
FH smokers exhibit a shift in haemostatic balance toward thrombosis, compared with FH non-smokers. Antoniades et al,35 demonstrated that FH smokers exhibited a decreased forearm vasodilatory response to reactive hyperaemia, increased inflammation and an imbalanced thrombosis/fibrinolysis equilibrium favouring hypercoagulation, compared with FH non-smokers.
Sebestjen et al,36 investigated biomarkers of hypofibrinolysis in FH individuals with and without CHD. They found significantly higher levels of tissue plasminogen activator (PA) antigen and PA inhibitor-1 antigen (both of which suppress fibrinolysis) in FH individuals with CHD. This shift of haemostatic balance from fibrinolysis toward hypercoagulation was independent of their LDL levels.
Plasma fibrinogen >300 mg/dL in homozygous FH (HoFH), heterozygous FH (HeFH) and non-FH individuals (non-FH) is associated with premature CHD (red). Plasma fibrinogen <300 mg/dL in HeFH and non-FH is associated with a lower incidence of CHD (blue).31 32 *Indicates p<0.05 compared with the relevant comparison group, based on statistical analyses in the original publication. CHD, coronary heart disease; FH, familial hypercholesterolaemia.
Non-lipid CHD risk factors
FH individuals are as susceptible to non-lipid CHD risk factors as non-FH individuals. The following is a subset of the literature that has documented this finding:
Galema-Boers et al 37 demonstrated that FH individuals with hypertension had more than twice the incidence of CHD than normotensive FH individuals, despite having equivalent LDL-C levels.
Miname et al 38 found that FH individuals with a high CAC score, which is a highly reliable marker of CHD, had significantly greater levels of fasting blood glucose than those with low CAC, but both groups had equivalent on-treatment levels of LDL-C.
Gaudet et al 39 reported that FH individuals with abdominal obesity and hyperinsulinemia exhibited a dramatically greater incidence of coronary artery disease (CAD) than FH individuals without abdominal obesity and hyperinsulinaemia, an effect which was independent of their LDL levels (figure 4).
Ye et al 40 found that patients with FH with detectable CAC had significantly increased high‐sensitivity C reactive protein (hsCRP) values and impaired flow-mediated dilation compared with FH patients without CAC, which was independent of LDL-C levels.
A subset of FH individuals display elevated TGs, in addition to increased LDL-C, a condition referred to as FH type IIb25 or familial combined hyperlipidaemia (FCH).41 The susceptibility of FCH individuals to develop CHD is influenced by genetics and obesity.42 43 FCH individuals exhibit a significantly higher rate of MI than FH individuals with low levels of TGs, despite having equivalent levels of LDL-C.25 Therefore, hypertriglyceridaemia and obesity, independent of LDL-C, in a subset of FH individuals increases their susceptibility to develop CHD.
Relative odds of coronary artery disease (CAD) in FH men according to upper (high) or lower (low) 50th percentile of waist circumference and fasting insulin concentration. Data from Gaudet et al. 39 *Indicates p<0.05 compared with the relevant comparison group, based on statistical analyses in the original publication FH, familial hypercholesterolaemia.
Cardiovascular risk biomarkers: summary
Almost a century ago, the finding that a subset of FH individuals developed premature CHD led to the assumption that it was their elevated total cholesterol (and later LDL-C), alone, that increased their susceptibility to develop CHD. However, more sophisticated, contemporary research has demonstrated that FH individuals are just as, and perhaps even more, susceptible to the same non-lipid risk factors that contribute to CHD as in the general population. Overall, these findings provide strong evidence that a subset of FH individuals are at increased risk of CHD because they are susceptible to diet, lifestyle, metabolic and genetic risk factors which are independent of their high LDL-C levels.
Influence of diet on heart disease risk biomarkers
Aside from the consensus on the hazards of excess sugar and trans fats consumption, there is a lack of agreement on how other dietary components influence the incidence of hard cardiovascular events, for example, stroke, MI or death. This state of uncertainty was addressed by DuBroff and de Lorgeril,13 who reported that dietary RCTs have rarely demonstrated significant benefits in hard cardiovascular outcomes. Moreover, objections have been raised against recommendations on restrictions of saturated fat consumption.8 9 12–14 44 Therefore, despite decades of research on diet and cardiovascular disease, there is little consensus on how consumption of different food categories contribute causally to the development of CHD.
An alternative approach to understanding how diet affects CHD risk is with the assessment of how macronutrients, specifically the ratio of carbohydrate to fat in the diet, affect surrogate biomarkers which are associated with the incidence of coronary events. This approach has been studied extensively in numerous RCTs which have assessed CHD-relevant biomarker changes in response to low carbohydrate diet (LCD) versus LFD. RCTs have demonstrated that the improvement in CHD biomarkers with LCD is equivalent, and in most measures superior, to biomarker modifications with an LFD.45–48
One example of a benefit of LCD on CHD risk is in the abatement of hypertension via a diet-mediated reduction of hyperinsulinaemia and hyperglycaemia. Specifically, hyperinsulinaemia in people with type two diabetes promotes renal sodium retention,49 which contributes to hypertension and myocardial hypertrophy.50 Two recent long-term clinical trials have shown that, over the course of 2 years, LCD resulted in significant and substantial reductions in blood pressure, as well as a reduction of hyperglycaemia and hyperinsulinaemia.51 52
Historically, Lp(a) has been viewed as a genetically determined marker of CHD risk which is unaffected by diet. However, this perspective on Lp(a) was based solely on studies conducted on individuals on an LFD, which does not affect Lp(a) or even increases Lp(a).53 LCD, by contrast, is the only dietary approach which has been shown to significantly reduce Lp(a) levels,46 an effect which may reduce the risk of CHD in FH (figure 2).
It is noteworthy that the basis of the diet-heart hypothesis was that consumption of food rich in saturated fat would increase risk for CHD. But in an RCT by Wood et al,46 subjects in the LCD group exhibited superior improvements in CHD risk factors than the LFD group, despite the LCD group having consumed more than three times as much saturated fat as the LFD group.
One final issue is whether FH individuals respond in an aberrant manner to LCD. Cole et al 54 assessed this issue by studying the effects of a moderately LCD (30%), high fat (55%) diet, supplemented with up to 1800 mg/day of cholesterol (from eggs), on serum lipids in FH subjects. These investigators reported that consumption of additional fat and cholesterol in the context of an LCD lowered TGs and raised HDL, and did not affect LDL-C levels in FH individuals. This study demonstrated that FH individuals responded to the low carbohydrate, high fat, high cholesterol diet in an equivalent manner to non-FH individuals.
In summary, the LCD has beneficial effects on well-established risk factors for CHD, including the ADRT components (sdLDL, HDL, TGs), Lp(a), body weight, inflammatory markers, fasting blood glucose, insulin levels and sensitivity, HbA1c and blood pressure.
Summary: evidence-based dietary recommendations for FH
Dietary recommendations for CHD prevention in FH individuals for the past eight decades have focused on targeting serum cholesterol reduction with a low saturated fat, low cholesterol diet. However, these recommendations are based largely on the antiquated and evidence-free diet-heart hypothesis. We have proposed that a revision of dietary recommendations for FH is justified, based on substantial evidence that the subset of FH individuals that develops CHD exhibits risk factors, such as enhanced thrombotic risk and a heightened sensitivity to risk factors associated with an insulin-resistant phenotype (elevated TGs, blood glucose, HbA1C, abdominal obesity, hyperinsulinaemia, hsCRP, low HDL, hypertension).
Our assessment of the literature is consistent with the conclusions of Gjuladin-Hellon et al,55 in their systematic review and meta-analysis on LCD and CHD risk: ‘Large RCTs of at least 6 months duration with carbohydrate restriction appear superior in improving lipid markers when compared with LFDs. … Dietary guidelines should consider carbohydrate restriction as an alternative dietary strategy for the prevention/management of dyslipidaemia for populations with cardiometabolic risk.’ Therefore, the evidence basis is sufficiently strong to provide the rationale for clinical trials to be conducted to determine if an LCD would prove to be effective in reducing the incidence of coronary events in FH individuals with an insulin-resistant phenotype or increased thrombotic risk.
Key points
Current dietary guidelines for management of coronary heart disease (CHD) risk in familial hypercholesterolaemia (FH) are based on the diet-heart hypothesis, which is outdated and unsupported.
There is no evidence to support the recommendation that FH individuals should consume a low saturated fat, low cholesterol diet.
A low carbohydrate diet (LCD) significantly improves cardiovascular disease biomarkers, compared with a low fat diet.
There is sufficient rationale for conducting clinical trials to assess the effects of an LCD on FH individuals with an insulin-resistant phenotype.
Extensive research has documented that hypercoagulation is a more important risk factor for CHD than low-density lipoprotein cholesterol in FH. Therefore, LCD trials should include FH subjects with an elevated risk of hypercoagulation.
Ethics statements
Patient consent for publication
Acknowledgments
We thank Robert DuBroff, Paul Leaverton and Sion Carter, as well as the anonymous reviewers, for their constructive editorial suggestions on a preliminary version of this paper. We dedicate this paper to our colleague, Paul Rosch, who contributed to a preliminary version of this paper before he passed away. Rosch was a staunch advocate of an evidence-based approach to cholesterol, heart disease and diet guidelines.
References
Footnotes
Twitter @LDLSkeptic, @Alabdulgaderaa
Contributors DMD wrote the first draft of this manuscript. All other authors provided critical reviews, commentaries and corrections.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. The open access publication cost was provided by the Western Vascular Institute, LTD., a not for profit organization and CrossFit, Inc.
Competing interests DMD is a member of the science advisory board for Anutra and has received honoraria from Pruvit. ZH receives royalties for books/content on diet and health. MK receives royalties for books on cholesterol and related medical issues. AAA donates royalties from his diet book to charity. UR receives royalties for books on diet and cholesterol. JV receives royalties for low-carbohydrate nutrition books. He is founder, consultant, and stockholder of Virta Health; a member of the advisory boards for Atkins Nutritionals Inc., UCAN, Ketone Sciences, and Axcess Global; and has received honoraria from Metagenics and Pruvit.
Provenance and peer review Not commissioned; externally peer reviewed.