Article Text
Abstract
Objectives To assess the beneficial and harmful effects of adding ivabradine to usual care in participants with heart failure.
Design A systematic review with meta-analysis and trial sequential analysis.
Eligibility criteria Randomised clinical trials comparing ivabradine and usual care with usual care (with or without) placebo in participants with heart failure.
Information sources Medline, Embase, CENTRAL, LILACS, CNKI, VIP and other databases and trial registries up until 31 May 2021.
Data extraction Primary outcomes were all-cause mortality, serious adverse events and quality of life. Secondary outcomes were cardiovascular mortality, myocardial infarction and non-serious adverse events. We performed meta-analysis of all outcomes. We used trial sequential analysis to control risks of random errors, the Cochrane risk of bias tool to assess the risks of systematic errors and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) to assess the certainty of the evidence.
Results We included 109 randomised clinical trials with 26 567 participants. Two trials were at low risk of bias, although both trials were sponsored by the company that developed ivabradine. All other trials were at high risk of bias. Meta-analyses and trial sequential analyses showed that we could reject that ivabradine versus control reduced all-cause mortality (risk ratio (RR)=0.94; 95% CI 0.88 to 1.01; p=0.09; high certainty of evidence). Meta-analysis and trial sequential analysis showed that ivabradine seemed to reduce the risk of serious adverse events (RR=0.90; 95% CI 0.87 to 0.94; p<0.00001; number needed to treat (NNT)=26.2; low certainty of evidence). This was primarily due to a decrease in the risk of ‘cardiac failure’ (RR=0.83; 95% CI 0.71 to 0.97; p=0.02; NNT=43.9), ‘hospitalisations’ (RR=0.89; 95% CI 0.85 to 0.94; p<0.0001; NNT=36.4) and ‘ventricular tachycardia’ (RR=0.59; 95% CI 0.43 to 0.82; p=0.001; NNT=212.8). However, the trials did not describe how these outcomes were defined and assessed during follow-up. Meta-analyses showed that ivabradine increased the risk of atrial fibrillation (RR=1.19; 95% CI 1.04 to 1.35; p=0.008; number needed to harm (NNH)=116.3) and bradycardia (RR=3.95; 95% CI 1.88 to 8.29; p=0.0003; NNH=303). Ivabradine seemed to increase quality of life on the Kansas City Cardiomyopathy Questionnaire (KCCQ) (mean difference (MD)=2.92; 95% CI 1.34 to 4.50; p=0.0003; low certainty of evidence), but the effect size was small and possibly without relevance to patients, and on the Minnesota Living With Heart Failure Questionnaire (MLWHFQ) (MD=−5.28; 95% CI −6.60 to −3.96; p<0.00001; very low certainty of evidence), but the effects were uncertain. Meta-analysis showed no evidence of a difference between ivabradine and control when assessing cardiovascular mortality and myocardial infarction. Ivabradine seemed to increase the risk of non-serious adverse events.
Conclusion and relevance High certainty evidence shows that ivabradine does not seem to affect the risks of all-cause mortality and cardiovascular mortality. The effects on quality of life were small and possibly without relevance to patients on the KCCQ and were very uncertain for the MLWHFQ. The effects on serious adverse events, myocardial infarction and hospitalisation are uncertain. Ivabradine seems to increase the risk of atrial fibrillation, bradycardia and non-serious adverse events.
PROSPERO registration number: CRD42018112082.
- heart failure
- cardiovascular diseases
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. All data in the systematic review will be made available upon reasonable request to the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Summary box
What is already known about this subject?
Ivabradine is recommended in patients with symptoms of heart failure despite optimal background therapy for reducing heart failure hospitalisation in the 2017 American guidelines on heart failure.
Ivabradine is recommended for reducing cardiovascular mortality and heart failure hospitalisation in the 2016 European guidelines on heart failure.
A recent Cochrane review did not find evidence of a difference between ivabradine and placebo/no intervention on cardiovascular mortality and serious adverse events.
Summary box
What are the new findings?
In our systematic review, including 109 randomised clinical trials with 26 567 participants, ivabradine did not seem to reduce all-cause mortality, cardiovascular mortality or myocardial infarction.
Ivabradine seemed to decrease the risk of serious adverse events, mainly due to a reduction in cardiac failure and hospitalisations, but these outcomes were poorly defined and poorly assessed.
The effect on quality of life was small and probably without relevance to patients.
Ivabradine seemed to increase the risk of atrial fibrillation, bradycardia and non-serious adverse events.
How might it impact clinical practice in the foreseeable future?
Based on the evidence, the guideline recommendations on the treatment of heart failure with ivabradine should be reconsidered.
Introduction
Of all deaths worldwide, 30% are attributable to cardiovascular disease.1 Heart failure is characterised by symptoms related to fluid retention such as peripheral oedema, breathlessness and dyspnoea.2 Heart failure can be caused by either functional cardiac disease (eg, a decrease in the function of the myocardium) or structural cardiac disease (eg, disease of the cardiac valves).3 4 Medical management of heart failure includes the use of beta-blockers, angiotensin receptor blockers, ACE inhibitors and diuretics (loop diuretics, thiazides and potassium-sparing diuretics). Ivabradine is a relatively new drug that was first introduced into heart failure guidelines in Europe in 2012 and in America in 2017.5 6
Ivabradine selectively inhibits the sinus node, thereby decreasing the heart rate. The decrease in heart rate, results in a decreased myocardial oxygen demand and an increased myocardial oxygen supply, thereby improving the mismatch seen in heart failure.7 Therefore, ivabradine might be an effective intervention in people with heart failure.7 8 A recently published Cochrane review assessed the beneficial and harmful effects of ivabradine in people with heart failure and included 19 trials with 19 628 participants and did not find evidence of a difference between ivabradine and control in regard to cardiovascular mortality and serious adverse events.9 Another systematic review included 10 trials with 18 036 participants, did not search all relevant databases, did not consider the risk of random error and did not assess the certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE).10 To the best of our knowledge, no previous systematic review has assessed the beneficial and harmful effects of ivabradine compared with usual care (ie, placebo or no intervention) for people with heart failure, searching all relevant databases while considering the risks of both systematic errors and random errors.9 11–15
Methods
We described our methodology in detail in our protocol that was published before conducting the literature search.2 16 We reported this systematic review according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.15 We included all trials comparing ivabradine with placebo or no intervention in patients with heart failure. Four authors (MM, EEN, S-HY and NL) independently searched and screened for trials published prior to 31 May 2021 in Medline, Embase, CENTRAL, LILACS, CNKI, VIP and other databases and trial registries, see supplement 1 in online supplemental file 1 for a detailed list of databases and trial registries. Detailed search strategies are presented in supplement 2 in online supplemental file 2. We included randomised clinical trials regardless of their design, the trial setting, the publication status, year, language or reporting of outcomes. Five authors (MM, EEN, NJS, NL and S-HY) worked in pairs and independently extracted data and assessed the risks of bias in the included trials. If data were missing or unclear, we attempted to contact the trial authors by email. We resolved disagreements through discussion or by consulting a third author (JCJ).2 We planned to include non-randomised studies identified during the literature search for the reporting of serious and non-serious adverse events. However, we did not identify such studies during the literature search, and we did not systematically search for such studies. Therefore, there is a risk that we have not identified and reported on all relevant serious and non-serious adverse events, especially those that are rare or only associated with long-term treatment.
Supplemental material
We predefined three primary outcomes: all-cause mortality, serious adverse events and quality of life. We also predefined three secondary outcomes and eight exploratory outcomes.2 We used the trial results reported at maximal follow-up for all our outcomes.
We predefined several subgroup analyses for the assessment of the primary outcomes:
Trials at high risk of bias compared with trials at low risk of bias
Men compared with women
Participants with a resting heart rate at or above 70 beats/min compared with below 70 beats/min.
Trials administering ivabradine at or above median daily dose compared with below median daily dose
Trials administering ivabradine at or above median duration compared with below median duration
Assessment of risk of bias
To assess the risks of systematic errors, we assessed the risk of bias for each included trial. The risk of bias was assessed individually by five reviewers working in pairs (MM, EEN, NJS, S-HY and NL).17 We assessed the risk of small study bias using funnel plots and funnel plot asymmetry tests. We planned to assess the risk of for-profit bias as part of the risk of bias assessment but post-hoc decided to only acknowledge for-profit bias throughout the review in line with the Cochrane Handbook.18
Assessment of statistical and clinical significance
We used Review Manager V.5.4 for all meta-analyses.19 We chose to analyse all primary and secondary outcome meta-analyses using fixed effect due to the BEAUTIFUL and the SHIFT trials accounting for more than 85% wt in all primary and secondary meta-analyses (excluding the quality of life assessment with the Minnesota Living With Heart Failure Questionnaire (MLWHFQ), see the Quality of life section).13 20 21 Random-effects meta-analyses were also performed as sensitivity analyses. We used trial sequential analysis to control random errors (see below) and we adjusted the thresholds for statistical significance, as suggested by Jakobsen and colleagues, to control for the risks of random errors.11 13 22 We used three primary outcomes and, therefore, adjusted the p value to 0.025 as the threshold for statistical significance. When analysing our secondary and exploratory outcomes, we used a p value of 0.05 as the threshold for statistical significance, since these outcomes were meant to be hypothesis generating.
For continuous outcome data, we converted medians and IQRs to means and SDs and we converted SEs to SDs. Continuous outcomes were reported using mean differences (MDs) with 95% CIs. Dichotomous outcomes were reported using risk ratios (RRs) with 95% CIs. We visually inspected forest plots for the presence of heterogeneity and quantified heterogeneity using I2 statistics. Meta-analyses results are presented in forest plots (see supplement 5 to 12 in online supplemental file 1).
Meta-analyses might include too few participants to obtain enough statistical power for the reliable assessment of intervention effects. Even with statistically significant results, the credibility is poor when too few participants are included, and the intervention effects may be overestimated or underestimated. Trial sequential analysis calculates the required information size (the number of participants) needed to confirm or reject predefined anticipated intervention effects.13 Furthermore, trial sequential analysis expands the CIs when the accrued information size has not reached the required information size. Trials included in meta-analyses might introduce heterogeneity, which is also accounted for in trial sequential analysis by increasing the required information size with increasing heterogeneity.11 In an empirical review, false positive results were present in 7 out of 100 of Cochrane meta-analyses with a total of 14 false-positive meta-analytic results. Trial sequential analysis would have prevented 13 of those, had it been implemented.23 Trial sequential analysis reduces the risk of false positive results and inaccurate effect estimates in systematic reviews of interventions.22 We reported the Trial Sequential Analysis adjusted-confidence intervals (CIs) that accounts for the uncertainty of the effect when the accumulating data in the meta-analysis had not yet reached the required information size. We also reported trial sequential analysis-adjusted CIs, if the cumulative Z-curve crossed any of the trial sequential analysis boundaries of either benefit, harm or futility.
To assess the impact of missing data, we used ‘best–worst case’ and ‘worst–best case’ analyses.17 We used GRADE to assess the certainty of evidence.24 25 We downgraded the certainty of evidence by two levels due to imprecision in GRADE if the accrued number of participants was below 50% of the diversity-adjusted required information size (DARIS) and by one level if the accrued number of participants was between 50% and 100% of DARIS. We did not downgrade if the cumulative Z-curve crossed the monitoring boundaries for benefit, harm or futility, or the DARIS was reached.
Results
From our literature search, we identified 4192 records. Additionally, 11 trials were identified from other sources. After the removal of duplicates, a total of 2539 records remained. We excluded a total of 2194 records based on their title or abstract. We excluded another 236 records based on their full text, see supplement 3 in online supplemental file 1. Therefore, we included a total of 109 clinical trials randomising 26 567 participants.20 21 26–132 Eighteen trials compared ivabradine with placebo20 21 26 27 44 55 56 63 68 70 72 74 76 82 91 93 94 118 and 91 trials compared ivabradine with ‘no intervention’. Of the 91 trials comparing ivabradine with ‘no intervention’, 48 trials used guideline-based therapy in both groups,28 30 32–36 38–40 48 51 60–62 64 66 67 69 73 75 77 78 80 84–87 89 92 95–99 101 103 109 112 113 115 116 120 122 123 125 128 132 37 trials used various beta-blockers at an equal dose in both groups other than guideline-based therapy,29 31 41 43 45–47 49 50 52–54 57–59 71 81 83 88 90 100 102 104 106–108 110 111 114 117 119 121 124 126 127 129 131 1 trial used cyclic AMP analogue other than guideline-based therapy,79 4 trials used levosimendan other than guideline-based therapy42 65 105 130 and 1 trial used trimetazidine other than guideline-based therapy.37 See online supplemental file 2, baseline characteristics.
Supplemental material
The BEAUTIFUL and the SHIFT trials contributed with more than 85% wt in all primary and secondary outcome meta-analyses.20 21 For both trials, we identified methodological limitations. First, neither of the trials were adequately registered prior to randomising the first participants in 2004 and 2006, respectively.20 21 133–136 Therefore, it was not adequately documented that the methodology used in the trials, including some outcomes and participating centres, was predefined. Second, primarily based on the results of these two trials, we found indications of a beneficial effect of ivabradine when assessing serious adverse events (see the Results section), primarily due to ivabradine decreasing the risk of ‘cardiac failure’ and ‘hospitalisations’ (see the Serious adverse events section). However, in the two trials, it was not described how ‘cardiac failure’ and ‘hospitalisation’ were assessed during follow-up or how ‘cardiac failure’ and ‘hospitalisation’ were defined. In the BEAUTIFUL trial, all-cause hospitalisation was not reported, which raises concerns of selective outcome reporting.20 Third, in the SHIFT substudy assessing quality of life using the Kansas City Cardiomyopathy Questionnaire (KCCQ), only 1944 participants (29.9%) of the 6505 participants analysed in the main trial were included.137 The reason was because some countries did not participate or did not have a translated version of the KCCQ, but otherwise it was unclear how this selection of participants was conducted.137 Fourth, for serious and non-serious adverse events, there were discrepancies between the data reported in the publication of the SHIFT trial when compared with the raw data presented on ClinicalTrials.gov, see supplement 11 in online supplemental file 1.21 135 The BEAUTIFUL and the SHIFT trials and its authors were sponsored by the company that developed ivabradine, but the trials were otherwise judged to be at low risk of bias. All other included trials were judged to be at high risk of bias, see online supplemental file 1, risk of bias. Due to these limitations, there is a risk that we overestimate the beneficial effects and underestimate the harmful effects of ivabradine.2 16 17
See supplement 4 online supplemental file 1 for risk of bias graph and summary.
Primary outcomes
All-cause mortality
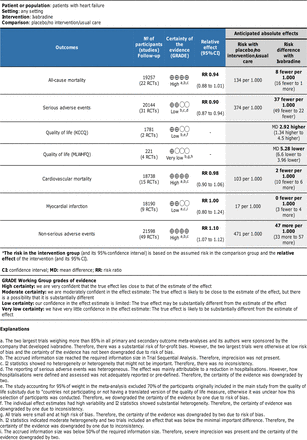
Two trial results were judged to be at low risk of bias (but at risk of for-profit bias).20 21 In trials at low risk of bias, mortality occurred in 1075 (12.3 %) of 8720 in the ivabradine groups compared with 1099 (12.6 %) of 8702 in the control groups. Meta-analysis showed no evidence of a difference between ivabradine and control on all-cause mortality (RR=0.98; 95% CI 0.86 to 1.10; I2=58%; figure 4 in online supplemental file 1). Meta-analysis of all trials showed a similar result (RR=0.94; 95% CI 0.88 to 1.01; p=0.09; 22 trials; high certainty of evidence; figure 6 in online supplemental file 1). Visual inspection of the forest plot and I2 statistics (I2=12%) indicated heterogeneity that might not be important. Trial sequential analysis showed that we had enough information to reject that ivabradine reduced the risk of all-cause mortality by 15% (RR=0.94; 95% CI 0.86 to 1.03; p=0.09; I2=16%; D2=61%; figure 8 in online supplemental file 1). This outcome result was judged to be at low risk of bias (but at risk of for-profit bias). Incomplete outcome data alone seemed to have the potential to influence the results. Visual inspection of the funnel plot and Harbord’s test (p=0.51) did not indicate funnel plot asymmetry. See summary of findings table (figure 1) and supplement 5 in online supplemental file 1.
Supplemental material
Summary of findings. RR, risk ratio. RCTs, randomised clinical trials. GRADE, Grading of Recommendations Assessment, Development and Evaluation.
Serious adverse events
Serious adverse events occurred in 3393 of 10 101 participants in the ivabradine groups compared with 3758 of 10 043 in the control groups. Meta-analysis showed evidence of a beneficial effect of ivabradine versus control on serious adverse events (RR=0.90; 95% CI 0.87 to 0.94; p<0.00001; 31 trials; number needed to treat (NNT)=26.3; low certainty of evidence; figure 17 in online supplemental file 1). Visual inspection of the forest plot and I2=37% indicated heterogeneity that might not be important. Trial sequential analysis showed that we had enough information to confirm that ivabradine decreased the risk of serious adverse events by 10% (RR=0.90; 95% CI 0.87 to 0.94; p<0.0001; I2=37%; D2=85%; Trial sequential analysis graph not produced due to the first trial exceeding the required information size). This outcome result was judged to be at high risk of bias. Incomplete outcome data alone did not seem to have the potential to influence the results. Visual inspection of the funnel plot and Harbord’s test (p=0.32) did not indicate funnel plot asymmetry. See Summary of findings table (figure 1) and supplement 6 in online supplemental file 1.
Individual serious adverse events
The 31 trials reported on 1033 individual serious adverse events. The majority of these serious adverse events were primarily reported in the BEAUTIFUL and the SHIFT trials. For all types of individual serious adverse events, we calculated RRs, 95% CIs and p values.
Ivabradine may decrease the risk of the following adverse events classified as serious by the trialists: cardiac failure (RR=0.83; 95% CI 0.76 to 0.90; p<0.00001; I2=41%; NNT=43.9; 5 trials), ventricular tachycardia (RR=0.59; 95% CI 0.43 to 0.81; I2=0%; NNT=212.8; 3 trials) and hospitalisation (RR 0.89; 95% CI 0.85 to 0.94; p<0.0001; I2=56%; NNT=37; 17 trials).
Ivabradine may increase the risk of bradycardia (RR=3.95; 95% CI 1.88 to 8.29; p=0.0003; I2=0%; number needed to harm (NNH)=303; 3 trials).
We regarded atrial fibrillation as a serious adverse event regardless of how it was reported in the included trials. Therefore, we conducted a meta-analysis, including the highest proportion of participants with atrial fibrillation as reported in the trials. Ivabradine may increase the risk of atrial fibrillation (RR=1.17; 95% CI 1.03 to 1.32; p=0.02; I2=0%; NNH=129.9; 10 trials).
Quality of life
Quality of life was reported using the KCCQ in two trials, including the SHIFT trial, analysing 1781 participants. Meta-analysis showed evidence of a beneficial effect of ivabradine versus control on quality of life using the KCCQ (MD=2.92; 95% CI 1.34 to 4.50; p=0.0003; low certainty of evidence; figure 27 in online supplemental figure 1). Visual inspection of the forest plot and I2=86% indicated substantial heterogeneity. Trial sequential analysis showed that we had enough information to confirm that ivabradine increased the quality of life by 2.92 points (TSA graph not produced due to the first trial exceeding the required information size). This outcome result was judged to be at high risk of bias. Incomplete outcome data seemed to have the potential to influence the results. We predefined that we would consider the observed SD divided by ‘2’ as the minimal important difference.2 In the trials using the KCCQ, the observed difference between ivabradine and control was 2.92 points at follow-up. The SD was approximately 16.8 points; hence, the minimal important clinical difference was 8.4 points. Therefore, the observed difference of 2.92 points at follow-up was only one-third of the minimal important difference.
Quality of life was reported using the MLWHFQ in 4 trials randomising 221 participants. In three trials, it was unclear whether SDs or SEs were reported and these were excluded from the analyses.33 80 92 Meta-analysis showed evidence of a difference between ivabradine and control on quality of life using the MLWHFQ (MD=−5.28; 95% CI −6.60 to −3.96; p<0.00001; very low certainty of evidence; figure 32 in online supplemental figure 1). Visual inspection of the forest plot and I2=35% indicated moderate heterogeneity. Trial sequential analysis showed that we had enough information to confirm MD of 5.28 points by ivabradine (MD=−5.28; 95% CI −7.32 to −3.24; p<0.0001; I2=35%; D2=52%; figure 34 in online supplemental figure 1). This outcome result was judged to be at high risk of bias. Incomplete outcome data alone did not seem to have the potential to influence the results. In the trials using MLWHFQ, the observed difference between ivabradine and control was 5.28 points at follow-up. The SD was 3.70; hence, the minimal important difference was 1.85 points. The observed difference of 5.28 points was above the minimal important difference. However, the evidence was very uncertain. See Summary of findings table (figure 1) and supplement 7 in online supplemental file 1.
Secondary outcomes
Cardiovascular mortality
Two trial results were judged to be at low risk of bias (but at risk of for-profit bias).20 21 In trials at low risk of bias, cardiovascular mortality occurred in 918 (10.6 %) of 8720 in the ivabradine groups compared with 926 (10.6%) of 8702 in the control groups. Meta-analysis showed no evidence of a difference between ivabradine and control on cardiovascular mortality (RR=0.99; 95% CI 0.86 to 1.15; p=0.91; I2=66%; figure 39 in online supplemental file 1). Meta-analysis of all trials showed showed a similar result (RR=0.98; 95% CI 0.90 to 1.06; p=0.58; 15 trials; high certainty of evidence; figure 41 in online supplemental file 1). Visual inspection of the forest plot and I2=7% indicated heterogeneity that might not be important. Trial sequential analysis showed that we had enough information to reject that ivabradine reduced the risk of cardiovascular mortality by 15% when compared with control (RR=0.98; 95% CI 0.88 to 1.08; p=0.58; I2=7%; D2=49%; figure 43 in online supplemental file 1). This outcome result was judged to be at low risk of bias (but at risk of for-profit bias). Incomplete outcome data alone did not seem to have the potential to influence the results. Visual inspection of the funnel plot and Harbord’s test (p=0.36) did not indicate funnel plot asymmetry. See Summary of findings table (figure 1) and supplement 8 in online supplemental file 1.
Myocardial infarction
Two trial results were judged to be at low risk of bias (but at risk of for-profit bias).20 21 In trials at low risk of bias, myocardial infarction occurred in 144 (1.7%) of 8709 in the ivabradine groups compared with 142 (1.6%) of 8690 in the control groups. Meta-analysis showed no evidence of a difference between ivabradine and control on myocardial infarction (RR=1.01; 95% CI 0.80 to 1.27; p=0.92; I2=0%; figure 49 in online supplemental file 1). Meta-analysis of all trials showed a similar result (RR=1.00; 95% CI 0.80 to 1.24; p=0.96; 9 trials; low certainty of evidence; figure 50 in online supplemental file 1). Visual inspection of the forest plot and I2=0% indicated no heterogeneity. Trial sequential analysis showed that we did not have enough information to reject that ivabradine reduced the risk of myocardial infarction by 15% when compared with control (RR=1.01; 95% CI 0.41 to 2.43; p=0.83; I2=0%; D2=0%; figure 52 in online supplemental file 1). This outcome result was judged to be at low risk of bias (but at risk of for-profit bias). Incomplete outcome data alone seemed to have the potential to influence the results. See Summary of findings table (figure 1) and supplement 9 in online supplemental file 1.
Non-serious adverse events
Two trial results were judged to be at low risk of bias (but at risk of for-profit bias).20 21 In trials at low risk of bias, non-serious adverse events occurred in 5264 (60.4%) of 8709 participants in the ivabradine groups compared with 4798 (55.2%) of 8690 participants in the control groups. Meta-analysis showed evidence of a harmful effect of ivabradine versus control on non-serious adverse events (RR=1.10; 95% CI 1.00 to 1.21; p=0.05; I2=93%; figure 57 in online supplemental file 1). Meta-analysis of all trials showed a similar result (RR=1.10; 95% CI 1.07 to 1.12; p<0.00001; NNH=22.5; 49 trials; high certainty of evidence; figure 59 in online supplemental file). Visual inspection of the forest plot and I2=12% indicated heterogeneity that might not be important. Trial sequential analysis showed that we had enough information to confirm that ivabradine increased the risk of non-serious adverse events by 10% when compared with control (RR=1.10; 95% CI 1.07 to 1.12; p<0.0001; I2=12%; D2=83%; figure 61 in online supplemental file 1). This outcome result was judged to be at low risk of bias (but at risk of for-profit bias). Incomplete outcome data alone did not seem to have the potential to influence the results. Visual inspection of the funnel plot and Harbord’s test (p=0.21) did not indicate funnel plot asymmetry. See Summary of findings table (figure 1) and supplement 10 in online supplemental file 1.
Individual non-serious adverse events
Ivabradine may increase the risk of ‘bradycardia’ (RR=1.62; 95% CI 1.01 to 2.60; p=0.05; I2=45%; NNH=39.4; 25 trials), ‘heart rate decreased’ (RR=4.32; 95% CI 3.39 to 5.50; I2=0%; NNH=33; 3 trials), and phosphenes (RR=4.71; 95% CI 3.67 to 6.04; p<0.00001; I2=0%; NNH=33.8; 20 trials).
Ivabradine may decrease the risk of ‘sinus tachycardia’ (RR=0.39; 95% CI 0.27 to 0.56; p<0.00001; NNT=52.4; 2 trials) and ‘hypotension’ (RR=0.70; 95% CI 0.55 to 0.90; I2=0%; NNT=192.3; 5 trials).
Exploratory outcomes
The results of our exploratory outcomes are reported in supplement 12 in online supplemental file 1.
Subgroup analyses
We predefined several subgroup analyses for the primary outcomes.2
When assessing all-cause mortality, test for subgroup differences (p=0.06) suggested a difference between trials administering ivabradine at or above median duration (RR=0.95; 95% CI 0.88 to 1.02) compared with trials administering ivabradine below median duration (RR=0.47; 95% CI 0.23 to 0.99).
When assessing serious adverse events, test for subgroup differences (p=0.005) suggested a difference between trials administering ivabradine at or above median duration (RR=0.92; 95% CI 0.88 to 0.95) compared with trials administering ivabradine below median duration (RR=0.53; 95% CI 0.36 to 0.77).
When assessing quality of life on the KCCQ, test for subgroup differences (p=0.007) suggested a potential difference between trials administering ivabradine at or above median duration (MD=2.40; 95% CI 0.77 to 4.03) compared with trials administering ivabradine below median duration (MD=12.00; 95% CI 5.23 to 18.77). When assessing quality of life on the MLWHFQ, test for subgroup differences (p=0.05) suggested a potential difference between trials administering ivabradine at or above median duration (MD=−13.80; 95% CI −23.17 to −4.44) compared with trials administering ivabradine below median duration (MD=−1.14; 95% CI −9.90 to 7.61).
See the respective supplementary sections for all-cause mortality, serious adverse events and quality of life for all subgroup analyses.
For all other subgroup analyses, test for subgroup differences did not show evidence of a difference between the subgroups or the subgroup analyses could not be conducted.
Discussion
The objective of our systematic review was to assess both the beneficial and harmful effects of adding ivabradine to usual care versus usual care with or without placebo in people with heart failure. We included 109 randomised clinical trials randomising 26 567 people with heart failure. All trials were judged to be at high risk of bias, except for the BEAUTIFUL and the SHIFT trials that were judged to be at low risk of bias (but at risk of for-profit bias).18 20 21 The BEAUTIFUL and the SHIFT trials accounted for more than 85% of weight in most meta-analysis and we did, therefore, now downgrade the certainty of the evidence due to risk of bias for most outcomes. However, we downgraded the certainty of the evidence for serious adverse events due to methodological limitations regarding the reporting of serious adverse events (see second paragraph of the Results section). Our results must be interpreted in the light of the high risks of bias and risks of for-profit bias that might result in overestimation of beneficial effects and underestimation of harmful effect of ivabradine. Due to the BEAUTIFUL and the SHIFT trials contributing with more than 85% of weight in all primary and secondary outcome meta-analyses, the results and conclusions presented in this systematic review can mostly be applied to people matching the populations in the BEAUTIFUL and the SHIFT trials.
Our results showed that ivabradine does not seem to affect the risks of all-cause mortality, cardiovascular mortality and myocardial infarction. Ivabradine seemed to decrease the risk of serious adverse events, primarily due to a decrease in the risk of ‘cardiac failure’, ‘hospitalisations’ and ‘ventricular tachycardia’. However, in the BEAUTIFUL and the SHIFT trials, and in the other trials reporting these outcomes, it was not described how these outcomes were assessed during follow-up or how the outcomes were defined. The effects on quality of life using the KCCQ were small and possibly without relevance to patients. The effects on quality of life using the MLWHFQ were very uncertain. Ivabradine seemed to increase the risk of atrial fibrillation, bradycardia, and non-serious adverse events. See Summary of findings table (figure 1).
Our systematic review has strengths. First, we predefined our methodology in detail in a protocol that was published prior to conducting the literature search.2 16 Second, we identified a total of 109 trials, which is more than any other previous systematic review on the topic. This has increased our precision and, therefore, strengthened our results. The recently published Cochrane review only identified 19 trials with 19 628 participants (90 trials less than ours).9 Third, we used trial sequential analysis on both primary and secondary outcomes11 and we adjusted our thresholds for statistical significance for the primary outcomes13 to control the risks of random errors. Fourth, we judged the risk of bias of all included trials to assess the risks of systematic errors.24 25 Fifth, we used our eight-step procedure to assess if the thresholds for statistical and clinical significance were crossed.13 Moreover, we included all randomised clinical trials identified through our literature search without imposing restrictions on their publication type, status, language and their reporting of outcomes. We attempted to contact the authors of the trials if data were incomplete or additional information was needed.
Our review also has limitations. First, all the included trials were judged to be at a high risk of bias as well as having a high risk of selective outcome reporting bias and for-profit bias.18 Nine of the trials were in some way sponsored by the company that developed ivabradine, including the BEAUTIFUL and the SHIFT trials that randomised 17 475 participants, accounting for more than 85% in all primary and secondary meta-analysis.20 21 55 63 70 74 81 93 Research has shown that drug trials funded by manufacturing companies tend to show more favourable efficacy results than trials funded by other sources.18 Moreover, 18 trials were reported only as abstracts which made the interpretation of methodology and results problematic.26 28–32 34 39 44 73 91 95 96 99 100 138–140 Therefore, there is a risk that our results are also biased and, therefore, overestimate the beneficial effects of ivabradine and underestimate the harmful effects.18 141–146
Conclusion and relevance
High certainty evidence shows that ivabradine does not seem to affect the risks of all-cause mortality and cardiovascular mortality. The effects on quality of life were small and possibly without relevance to patients on the KCCQ and were very uncertain for the MLWHFQ. The effects on serious adverse events, myocardial infarction and hospitalisation are uncertain. Ivabradine seems to increase the risk of atrial fibrillation, bradycardia and non-serious adverse events,
Differences between the protocol and the systematic review
We conducted our literature search in parallel with another systematic review on the effects of adding ivabradine to usual care in participants with angina pectoris due to coronary artery disease.147 We originally planned to analyse and report the results, including participants with coronary artery disease and participants with heart failure into one review, but due to clinical and statistical heterogeneity, we decided to report the results in two separate reviews.2
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. All data in the systematic review will be made available upon reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Acknowledgments
We would like to thank Liliya Ziganshina for assisting us with screening and data extracting articles in Russian.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Guarantors: MM and JCJ.
MM: conceived the systematic review, conducted literature search, data extraction, data analysis and data interpretation and wrote the article. EEN, NL and S-HY: conducted literature search and data extraction and amended the article. NJS: conducted data extraction and amended the article. CG: helped conceive the systematic review, provided invaluable comments and amended the article. JCJ: conceived the systematic review, aided in data interpretation and amended the article.
Funding This study was funded by the Copenhagen Trial Unit, Centre for Clinical Intervention Research, through wages paid to JCJ and CG. The funding is through the Danish Finance Act and the funding source had no influence on the systematic review.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.