Article Text
Abstract
A systematic review identifies, appraises and synthesises all the empirical evidence from studies that meet prespecified eligibility criteria to answer a specific research question. As part of the appraisal, researchers use explicit methods to assess risk of bias in the results’ from included studies that contribute to the review’s findings, to improve our confidence in the review’s conclusions. Randomised controlled trials included in Cochrane Reviews have used a specific risk of bias tool to assess these included studies since 2008. In 2019, a new version of this tool, Risk of Bias 2 (RoB 2), was launched to improve its usability and to reflect current understanding of how the causes of bias can influence study results. Cochrane implemented RoB 2 in a phased approach, with users of the tool informing guidance development. This paper highlights learning for all systematic reviewers (Cochrane and non-Cochrane) from the phased implementation, highlighting differences between the original version of the tool and RoB 2, consideration of reporting systematic review protocols or full review reports that have used RoB 2, and some tips shared by authors during the pilot phase of the implementation.
Data availability statement
No data are available. Not applicable.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Assessing risk of bias in the results of included studies of a systematic review is a key step in ensuring our confidence in the review’s findings. A new tool for assessing risk of bias in the results from randomised controlled trials was launched in 2019 called Risk of Bias 2 (RoB 2). During the phased implementation of RoB 2 in Cochrane, guidance for authors was developed.
WHAT THIS STUDY ADDS
This paper details the differences between the original risk of bias tool and RoB 2, as well as guidance developed by Cochrane for authors on using RoB 2 in systematic reviews that include randomised controlled trials which can be applied to non-Cochrane systematic reviews.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This paper provides recommendations for reporting systematic review protocols or full review reports that have used RoB 2, along with additional tips shared by Cochrane authors, to guide authors of any systematic review that includes randomised controlled trials and wants to use RoB 2 to assess the risk of bias in the studies’ results.
Introduction
A systematic review identifies, appraises and synthesises all the empirical evidence from studies that meet prespecified eligibility criteria to answer a specific research question. Researchers conducting systematic reviews seek to use explicit, systematic methods that are selected with a view aimed at minimising bias and imprecision to inform decision making. Assessing risk of bias in the design, conduct and reporting of the included studies in the review is a key step. This helps systematic reviewers, and the users of these reviews, to understand whether there is any risk of bias in the included study’s results that could distort the review’s results. This helps improve our confidence in the review’s conclusions and that it accurately represents the truth (that the true effect of the intervention has not been overestimated or underestimated).
Cochrane defines bias in this context as ‘a systematic error, or deviation from the truth, in results’.1 Before the mid-2000s, tools that critically assessed studies within reviews typically considered the broader notion of ‘methodological quality’ which often involved a combination of risk of bias, imprecision, relevance, applicability, ethics and completeness of reporting. However, this breadth of concepts was not based on any particular underlying theoretical framework for ‘quality’ assessment and therefore different tool resulted in different assessments for the same study.2 To address this, in 2008, Cochrane released a risk of bias tool to focus on the single concept of risk of bias in randomised controlled trials (RCTs) included within its reviews of interventions.3 Other concepts are now considered at other stages in the review process. For example, precision (the extent to which results are free of random errors) and external validity (directness, applicability or generalisability) are domains of GRADE (Grading of Recommendations, Assessment, Development and Evaluations).4
In 2019, a new version of the tool, Risk of Bias 2 (RoB 2), was launched to improve its usability, address some of the limitations of the original version5 6 and to reflect current understanding of how the causes of bias can influence study results.7 8 A recent study by Minozzi et al 9 found that 69% (95/137) reviews published between 2019 and 2021 adhered to RoB2 by assessing an outcome rather than the study. However, the majority of these reviews only included a single primary outcome and when the analysis was restricted to reviews with at least two primary outcomes, adherence decreased to 29%.
Cochrane used a phased implementation approach to introduce RoB 2 to authors of its systematic reviews. It aimed for a supported and gradual roll-out to observe and address common issues as they arose.8 Following the publication of the first Cochrane Review from the pilot phase,10 RoB 2 is now the recommended tool for assessing risk of bias in RCTs in Cochrane Reviews, though the original risk of bias tool is still acceptable (https://community.cochrane.org/news/status-and-expectations-implementation-risk-bias-2-cochrane-intervention-reviews).
Over 150 Cochrane Reviews are now in development that use RoB 2. In this paper, the team that led the implementation and members of the Cochrane Bias Methods Group share the guidance developed to support authors using RoB 2 in systematic reviews.
Online supplemental appendix 1 details the key resources, training, tools, software and template recommended by Cochrane that are available for systematic review authors to help them learn about and use RoB 2.
Supplemental material
Differences between the original risk of bias tool and RoB 2
A comparison of the differences between the two tools can be seen in table 1. One key difference between the two is that risk of bias assessments for RoB 2 are more explicitly associated with a specific result for an outcome from each included study (result-level assessment).
Differences between the original risk of bias tool and RoB2 for assessing randomised controlled trials
This recognises that different outcomes from one study could have different issues that affect the risk of bias, for example, an objective outcome (eg, maximal cardiorespiratory fitness) versus a subjective outcome (eg, health-related quality of life) as seen in Williams et al 10 Cochrane Review on physical activity interventions for people with congenital heart disease. For one of the studies (‘Sandberg 2018’), the authors judged the health-related quality of life result to be of high risk of bias because the participants both reported the outcome and were aware of the intervention, and they felt that this knowledge would have likely influenced the outcome (RoB 2, domain 4; blinding of the outcome assessment). In contrast, they judged this same domain in the same study to be at low risk of bias for the maximal cardiorespiratory fitness result because most of the assessors were blinded and for those who were not blinded, the authors felt that this knowledge would not have influenced the measurement as it is an objective outcome.
In addition, RoB 2 recognises that different results for the same outcome from one study could have different issues that affect the risk of bias. As an example, a Cochrane Review by Kew et al 11 on increasing the dose of inhaled corticosteroids when asthma symptoms worsen to reduce the need for further treatment included the outcome of treatment failure. This outcome was assessed in two populations; all randomised participants and those participants who started their study inhaler. The result for each outcome (treatment failure in all randomised participants vs treatment failure in only those who took their study inhaler) were different and for each result there were different issues that affected the risk of bias, as seen in figure 1. For one of the studies (‘Oborne 2009’), the authors judged the treatment failure in all randomised participants result to be of low risk of bias because all participants contributed data for the intention-to-treat analysis up to the point at which they left the study (RoB 2 domain 3; missing outcome data). In contrast, they judged this same domain in the same study to be of high risk of bias for the treatment failure in only those who took their study inhaler result as this population represents a relatively small, non-randomised proportion of the full cohort, there was an imbalance in the number of people in each group who took the study inhaler, and patients who discontinued who did not use the study inhaler might have done so for reasons relating to their disease worsening. Another hypothetical example is a systematic review interested in a outcome measured at end of treatment, such as 4 weeks, and at longest follow-up, such as months or years later. These two results for the same outcome may theoretically have different risk of biases, that is, a lower risk of bias at end of treatment and higher risk of bias at longest follow-up due to missing outcome data.
Example of how different results from the same outcome from one study or the effect of interest (intention to treat vs per-protocol) can have implications on risk of bias assessments using RoB 2.11 ROB 2, Risk of Bias 2.
Another important development is that RoB 2 requires authors to specify whether the result being assessed is being interpreted as the effect of assigning participants to interventions (the intention-to-treat effect) or the effect of participants adhering to their assigned intervention according to the trial protocol (the per-protocol effect). Authors need to think carefully about the aim of their systematic review, and which is most appropriate for each result. The effect of interest will have implications for the risk of bias due to deviations from the intended intervention (RoB 2, domain 2). An example of this is again seen in Kew et al Cochrane Review11 where, in the included trials, the randomised population only starts the intervention at the onset of treatment failure. The critical outcome for the review was treatment failure in all randomised participants (the intention-to-treat effect), and an important secondary outcome was treatment failure in participants using the study inhaler (the per-protocol effect); the same outcome in different populations resulted in different effects of interest and different risk of bias assessments (see figure 1).
By considering the specific result for each outcome of interest from a study separately, we can more accurately assess bias for those results which will increase our confidence in the evidence presented in the systematic review.
Richter and Hemmingsen12 compared RoB 2 to the original tool and found that the mean assessment times were comparable and there were few difficulties achieving agreement between raters with both tools. The biggest divergence between the tools was with subjective outcomes in open-label studies, where the original tool was more likely to penalise than RoB 2. The original tool led to harsher judgements due to the options available (high/low/unclear risk) whereas the signalling questions and guidance made RoB 2 easier to work through complexity and context.
This paper concentrates on guidance for parallel RCTs only. The RoB 2 tool has two supplemental variants, one for cluster RCTs and one for crossover RCTs, both available from the Risk of Bias tools website (https://www.riskofbias.info/welcome/rob-2-0-tool).
RoB 2 considerations for systematic review protocol development
It is important to consider carefully at the protocol stage how RoB 2 will be used in a review. As RoB 2 is a result-level tool and does not need to be completed for all the outcomes and all the studies in the systematic review, prioritisation of which outcomes to assess is likely to be needed. Without considering this, reviewers can increase their workload substantially and are at risk of using the tool incorrectly, that is, with a study-level focus instead of the correct results-level focus. Cochrane recommends that review authors limit the RoB 2 assessments to the results for their outcomes planned for inclusion in a summary of findings table, that is, those outcomes deemed to be the most critical and important outcomes for decision making. Table 2 details these considerations.
10 items Cochrane’s evidence production and methods directorate recommends authors consider and report when developing a protocol for a systematic review that plans to use the RoB 2 tool
Rows highlighted green are core to the context of a systematic review and must be prespecified to facilitate the accurate use of RoB 2 during risk of bias assessments. Includes guidance from chapter 71 and chapter 8 of the Cochrane Handbook,13 as well as PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020.14
RoB 2 considerations for reporting the full systematic review
Table 3 details the considerations needed when reporting the full systematic review.
Seven items Cochrane recommends authors report in a completed systematic review that used the RoB 2 tool
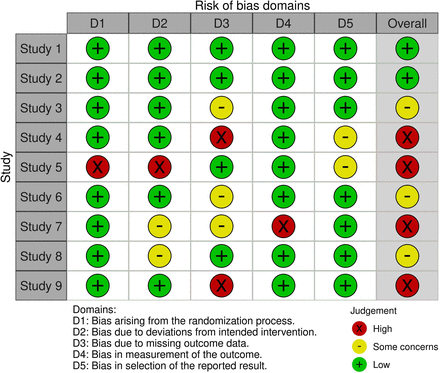
Example of a risk of bias figure for one result for one outcome. Generated using robvis software.20
Note that this checklist only highlights RoB 2 considerations for review reporting. Rows highlighted green are core to understanding the risk of bias assessments in the context of a systematic review’s conclusions. Some journals may have specific guidance on use of tables and figures, so this guidance may need to be adapted to meet those journal requirements. Includes guidance from chapter 7 and chapter 14 of the Cochrane Handbook.1 4
RoB 2 tips
In this section, we bring together some of the key takeaways from the RoB 2 pilot phase of implementation.
Create a RoB 2 decision tool following protocol development
While RoB 2 is a result-based assessment, considering which domains are expected to be consistent across results within a study and designing the data collection form accordingly can save a lot of time. This early investment goes a long way. Some teams have created a risk of bias decision tool that is specific to their review, to help reviewers make consistent decisions and to ease the process of assessing bias, for example, issues in randomisation will be common to all outcomes, issues of missing data may differ for outcomes at different time points, and issues of outcome assessment may be different between patient-reported outcomes and outcomes derived from routine data sources.15 The first few assessments may take some time to get right but once done, subsequent assessments naturally become much easier and faster.
Disagreements are no bad thing
Practising a couple of assessments will always highlight differences that can be ironed out, but inter-rater discrepancies beyond that should be expected and may even improve the review. The signalling questions in RoB 2 provide a clearer framework for discussing differences in judgements and justifications than the old tool, and the process of doing so is a key part of gaining understanding and interrogating the evidence.
Back to bias assessment as it was always intended
Shifting from assessing studies to assessing results may initially feel like a daunting task but, once a rhythm is found, it can refocus the mind on why bias assessment is so important in systematic reviews. RoB 2 provides a framework for building meaningful bias considerations through reviews, from protocol planning to writing up results and implications for practice.
Authors are not expected to assess risk of bias for all results from all included studies
The risk of bias assessment should focus on results of studies that contribute information to outcomes that users of the review will find most useful. This will generally correspond to the results that are used to populate outcomes in summary of findings tables; however, this will depend on the review question and protocol, which may have specified other outcomes for risk of bias assessment.
Conclusions
RoB 2 is the recommended tool to assess the risk of bias of specific results included in a systematic review. It has some fundamental differences from the original Cochrane risk of bias tool and these need to be understood before the tool is applied. This includes whether the authors are interested in the effect of assignment or adherence and which results they plan to assess. Prespecifying some items from RoB 2 can aid implementation of the tool. Transparency is key and all supporting material should be submitted alongside the full review and for peer review. An Excel tool is available on the risk of bias website (www.riskofbias.info) to facilitate completion of the assessments. Assessing the risk of bias for specific results allows us to see the impact this has on a synthesis or meta-analysis, whereas previously a study was usually judged for bias overall and might have left some results with a worse assessment of bias than was warranted.
Data availability statement
No data are available. Not applicable.
Ethics statements
Patient consent for publication
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @dwan_kerry
EF, THM and KD contributed equally.
Contributors KD conducted the study by leading the RoB2 pilot and wrote the guidance, commented on the manuscript, submitted the manuscript and acts as guarantor for the manuscript. EF conducted the study by leading the RoB2 pilot and wrote the guidance and this manuscript. THM conducted the study, wrote the guidance and commented on the manuscript. JPTH was involved in the conduct of the study, contributed towards the guidance and commented on the manuscript. IB was involved in the conduct of the study, contributed towards the guidance and commented on the manuscript. AH was involved in the conduct of the study, contributed towards the guidance and commented on the manuscript. CHN commented on the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests EF is employed by Cochrane. During the development of the manuscript, KD and THM were employed by Cochrane. JPTH, IB, AH and CHN are leads of the Cochrane Bias Methods Group. JPTH, IB and AH were part of the development team for the RoB 2 tool.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.